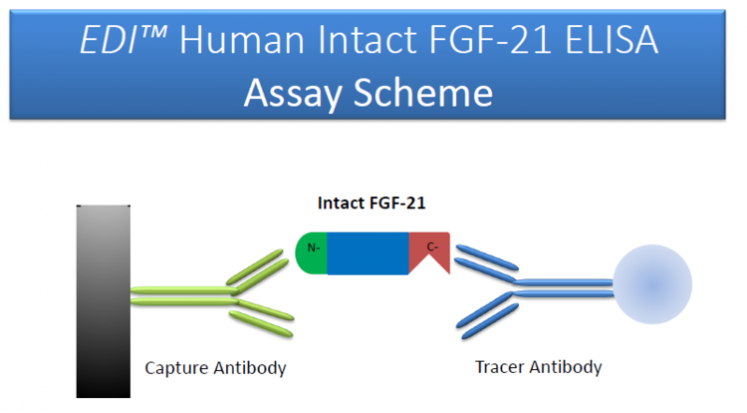
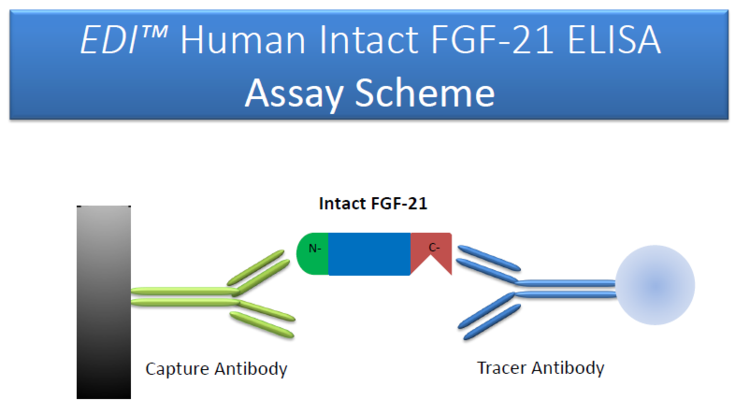
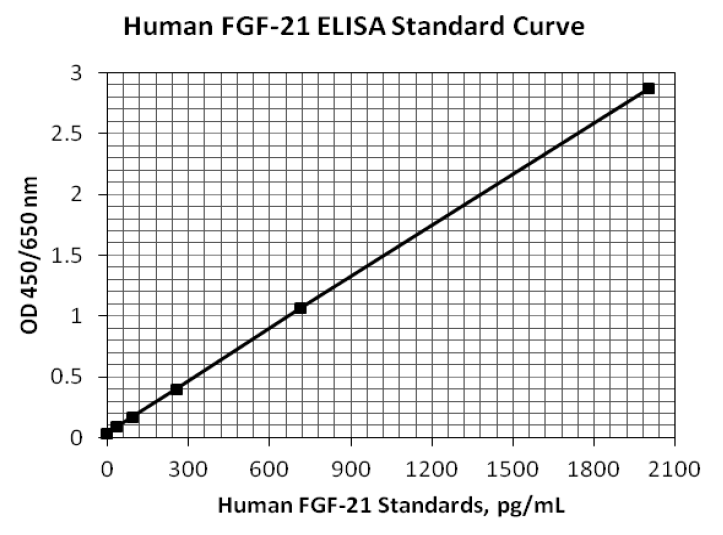
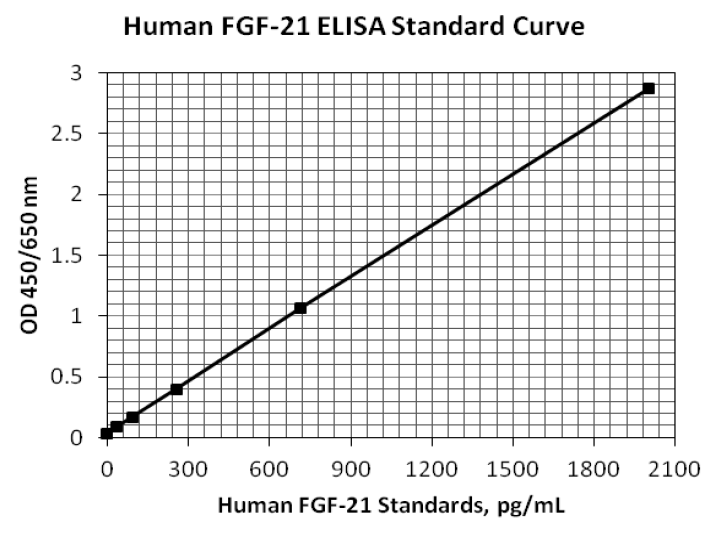
Background
Fibroblast growth factor 21 (FGF-21) belongs to the FGF-19 subfamily, which includes FGF-19, FGF-21, and FGF-23. The FGF-19 family members are potent endocrine hormones in the regulation of a diverse physiological homeostasis.
The intact FGF-21 is a small protein comprising of 181 amino acids. Administration of recombinant FGF-21 lowered plasma glucose and insulin levels, reduced hepatic and circulating triglyceride and cholesterol levels, and improved insulin sensitivity, energy expenditure, hepatic steatosis, and obesity in a range of insulin-resistant animal models. The physiological functions of FGF-21 rely on the intact molecular structure and animno acid sequence in its N-terminal and C-terminal region. An N-terminal-truncated FGF-21 (7-181) is a potent inhibitor that competitively inhibits the biological activity of intact FGF-21 (1-181). Therefor, it is important to measure the amount of circulating, intact FGF-21 levels in the assessment of physiological and pathophysiological condition. An assay that determines FGF-21 fragments may overestimate the biological activity of the protein in test samples.
Circulating FGF-21 is a biomarker and its levels are increased in patients with nonalcoholic fatty liver disease (NAFLD), type 2 diabetes, gestational diabetes, and obesity. An increase of circulating FGF-21 is also found in patients with Cushing's syndrome and patients with lipodystrophy induced by HIV-1, as well as those with chronic renal disease or end-stage renal disease (ESRD).