Background
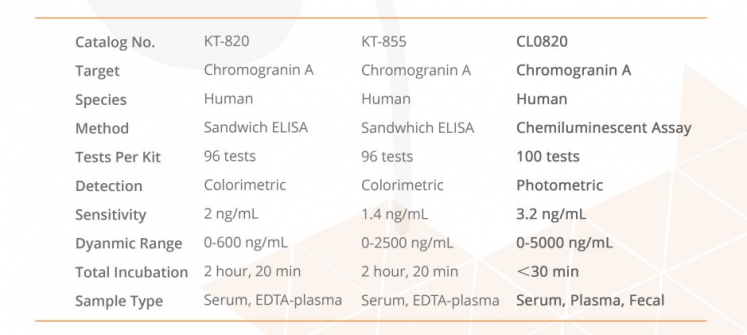
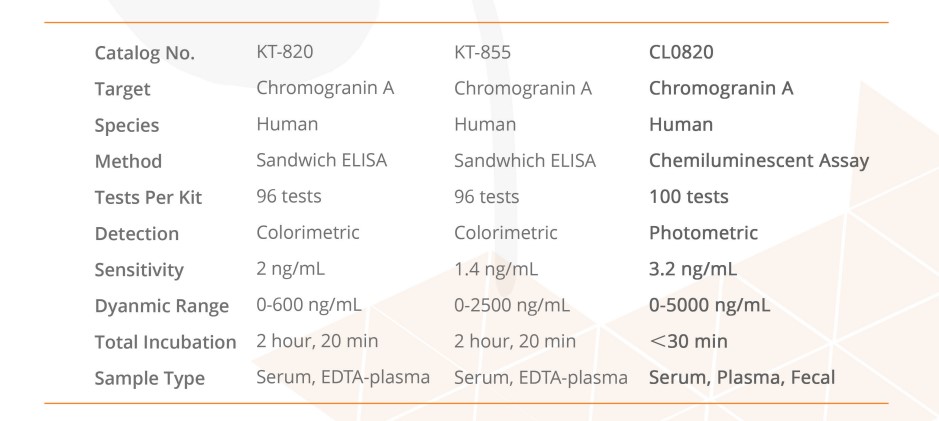
This ELISA is designed, developed and produced for the quantitative measurement of human chromogranin A in EDTA-plasma or serum sample. The assay utilizes the two-site “sandwich” technique with two selected antibodies that bind to different epitopes of human chromogranin A. Assay calibrators, controls and patient samples are added directly to wells of microplate that is coated with a polyclonal chromogranin A antibody. After the first incubation period, the antibody on the wall of microtiter well captures human chromogranin A in the sample and unbound protein in each microtiter well is washed away. Then a horseradish peroxidase (HRP)-labeled monoclonal anti-human chromogranin A antibody is added to each microtiter well and a “sandwich” of “monoclonal antibody – human chromogranin A – polyclonal antibody” is formed. The unbound monoclonal antibody is removed in the subsequent washing step. For the detection of this immunocomplex, the well is incubated with a substrate solution in a timed reaction and then measured in a spectrophotometric microplate reader. The enzymatic activity of the immunocomplex bound to the chromogranin A on the wall of the microtiter well is directly proportional to the amount of chromogranin A in the sample. A calibration curve is generated by plotting the absorbance versus the respective human chromogranin A concentration for each calibrator on point-to-point or cubical scales or 4 parameter curve fits. The concentration of human chromogranin A in test sample is determined directly from this calibration curve.