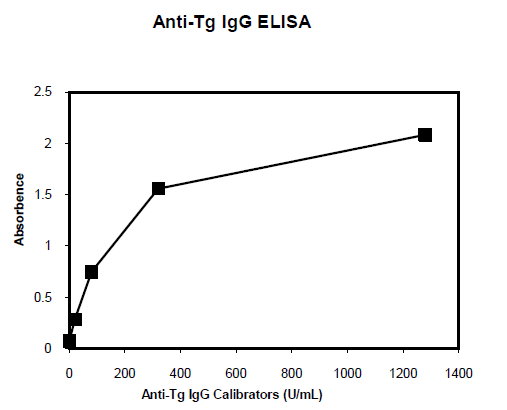
Background
Measuring serum autoantibodies to thyroglobulin (Tg) and thyroid peroxidase (TPO) to aid in the detecting and monitoring of autoimmune thyroid disease is a routine practice. Serum anti-TPO autoantibody and anti-Tg autoantibody are fund to be well-correlating with histological changes in Hashimoto's thyroiditis. Clinically, positive anti-TI autoantibody is detected in patients with chronic thyroiditis (70-90%), primary hypothyroidism (~60%), thyrotoxicosis (~50%), and thyroid tumors (~17%). Anti-Tg autoantibodiy is mainly identified in patients with Hashimoto's thyroiditis and Graves' disease (40-70%).
Although ELISA technology has applied to detecting these autoantibodies, the high background in normal populations decrease the clinical diagnostic sensitivity and speicificity. This highly sensitive EDI™ anti-Tg autoantibody ELISA kit was developed with proprietary technology that leads to a very low reaction background in normal populations, and thus increases the clinical diagnostics sensitivity and specificity.