Background
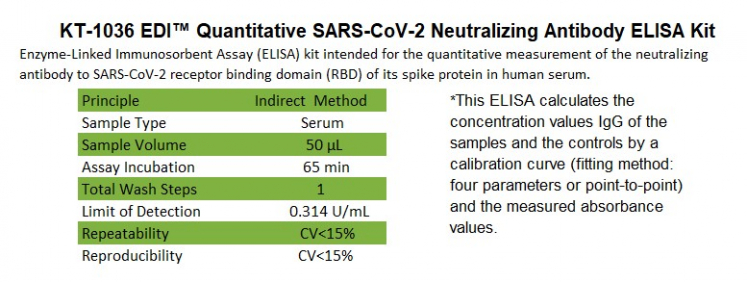
This ELISA kit is designed, developed, and produced for the quantitative measurement of neutralizing antibodies to SARS-CoV-2 receptor binding domain (RBD) of its spike protein in human serum This assay utilizes microplate-based enzyme immunoassay technique. Assay calibrators, controls, and human serum samples are added to the microtiter wells of a microplate coated with streptavidin. Simultaneously, horseradish peroxidase (HRP) labeled COVID-19 recombinant spike protein and biotinylated angiotensin converting enzyme-2 (ACE-2) are added to each well. After the first incubation period, the unbound protein matrix is removed with a subsequent washing step; a complex of "Streptavidin---Biotin-ACE-2---HRP-COVID-19 recombinant spike protein" is formed. If there is specific COVID-19 neutralizing antibody present in a tested specimen, the formation of the above complex is blocked. A color reaction with a substrate solution in a timed reaction is measured in a spectrophotometric microplate reader. The HRP enzymatic activity of the complex on the wall of the microtiter well is inversely proportional to the amount of the COVID-19 neutralizing antibody level in the tested specimen.