Background
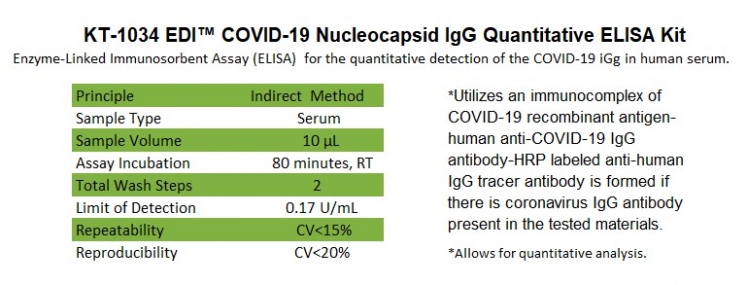
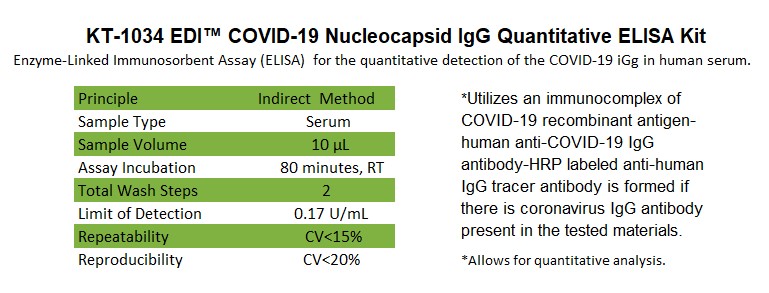
This ELISA kit is designed, developed, and produced for the qualitative measurement of human anti-COVID-19 IgG antibody in serum. This assay utilizes the microplate based enzyme immunoassay technique. Assay calibrators, controls, and 1:100 diluted human serum samples are added to the microtiter wells of a microplate that was coated with COVID-19 recombinant full length nucleocapsid protein. After the first incubation period, the unbound protein matrix is removed with a subsequent washing step. A horseradish peroxidase (HRP) labeled polyclonal goat anti-human IgG tracer antibody is added to each well. After an incubation period, an immunocomplex of "COVID-19 recombinant antigen – human anti-COVID-19 IgG antibody - HRP labeled anti-human IgG tracer antibody" is formed if there is specific coronavirus IgG antibody present in the tested specimen. The unbound tracer antibody is removed by the subsequent washing step. HRP-labeled tracer antibody bound to the well is then incubated with a substrate solution in a timed reaction and then measured in a spectrophotometric microplate reader. The enzymatic activity of the tracer antibody bound to the anti-COVID-19 IgG on the wall of the microtiter well is proportional to the amount of the anti-COVID-19 IgG antibody level in the tested specimen.