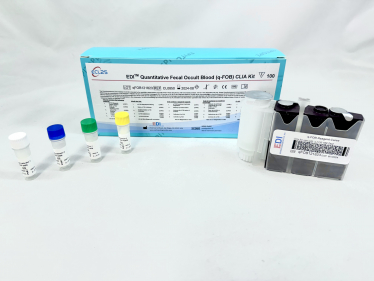
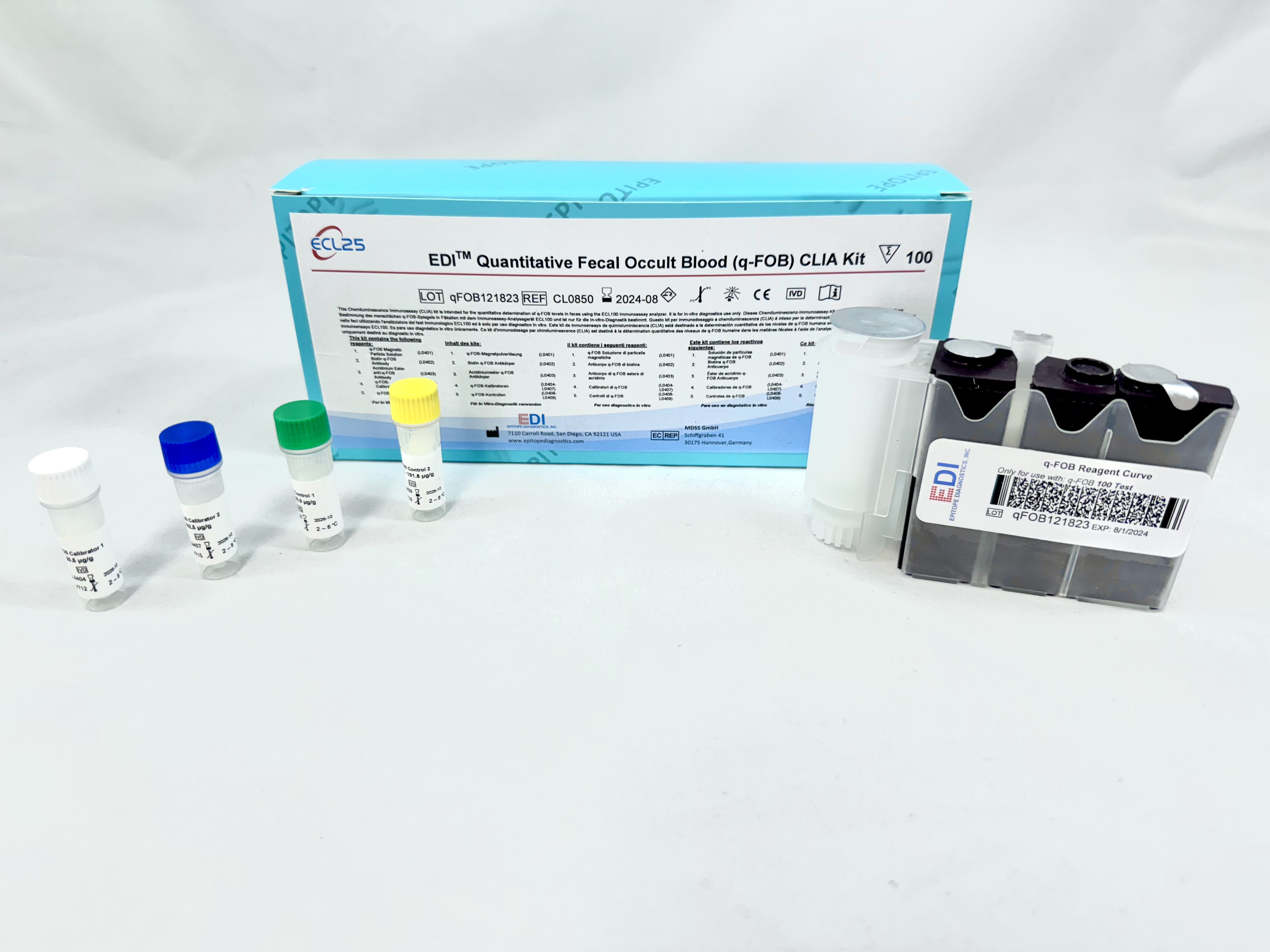
Background
This CLIA is designed, developed, and produced for the quantitative measurement of q-FOB level in fecal samples. The assay utilizes a two-site “sandwich” technique with two antibodies that bind to different epitopes of hemoglobin.
Assay calibrators, controls, or patient samples are added directly to a reaction vessel containing streptavidin coated magnetic particles. Simultaneously, anacridinium ester antibody and a biotin antibody are added. The magnetic particles capture the biotin antibody as well as an immuno complex in the form of “magnetic particles – biotin hemoglobin antibody –hemoglobin – acridinium ester hemoglobin antibody”.
The materials bound to the solid phase are held in a magnetic field while unbound materials are washed away. Then, the trigger solution is added to the reaction vessel and light generated by the reaction is measured with the ECL100 or ECL25 analyzer. The relative light units (RLU) are proportional to the concentration of hemoglobin in the sample. The amount of analyte in the sample is determined from a stored, multi�point calibration curve and reported in serum hemoglobin concentration.