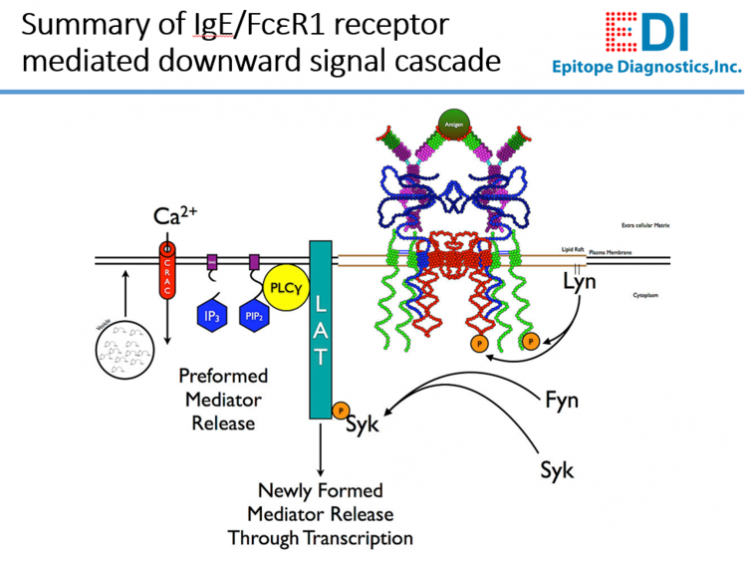
Background
The presence of anti-IgE receptor (FcεR1α) antibodies in patient serum or plasma has been associated with chronic spontaneous urticaria (CSU), which is a common skin disorder affecting 0.5% to 1.8% of the general population. It is characterized by repeated occurrence of short-lived cutaneous wheals accompanied by redness and itching. Although the CSU symptoms are very much similar to those of acute urticaria triggered by allergens, in most CSU cases, there is no definite identifiable and direct external triggering factor.
The pathogenesis of CSU has not yet been fully elucidated, but a proportion of patients with CSU have been found to have functional autoantibodies. In CSU patients, circulation human anti-FcεR1α autoantibodies are seen in 30% to 60% and anti-IgE autoantibodies in 5% to 10%. These human autoantibodies are mainly IgG subtype.