New Version of Fecal Sample Collection Tube!
September 19, 2022
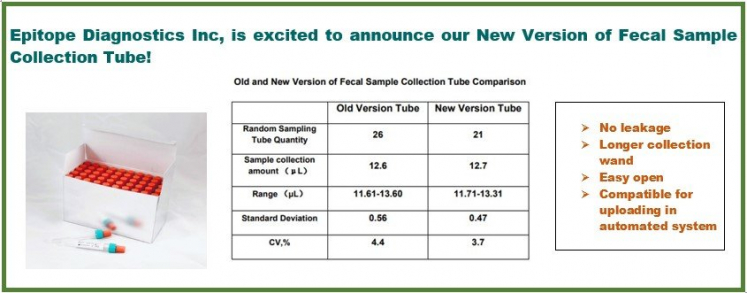
Fecal sample is used for many clinical laboratories test in disease diagnosis and monitoring. The most popular tests include Fecal Occult Blood test, fecal Calprotectin test, fecal Pancreatic Elastase 1 test, fecal Helicobacter pylori antigen test, fecal Giardia and cryptosporidium antigen tests, etc. However, unlike serum and plasma-based test, fecal sample requires collection and extraction before testing. The tradition way of accurate fecal sample collection extraction involves weighing stool on an analytical scale and extracted with a volume of buffer. Epitope Diagnostics has invented a Stool Sample Quantitative Collection and Extraction Device (sQED, US Patent issued). This sQED provide convenient and accurate collection and extraction of stool specimen without the traditional weighing process. Moreover, this device standardizes the collection process and the extracted sample can be directly used for automated immunoassay system.